Learn About 5 Scary Consequences to Ocean Acidification
We’ve all heard it before: climate change is affecting our planet. And not in a good way.
But most of what we hear, is about how climate change will affect our atmosphere.
In this article, we’ll cover a less-discussed, but just-as-important climate change issue: ocean acidification.
Ready?
Let’s get started!
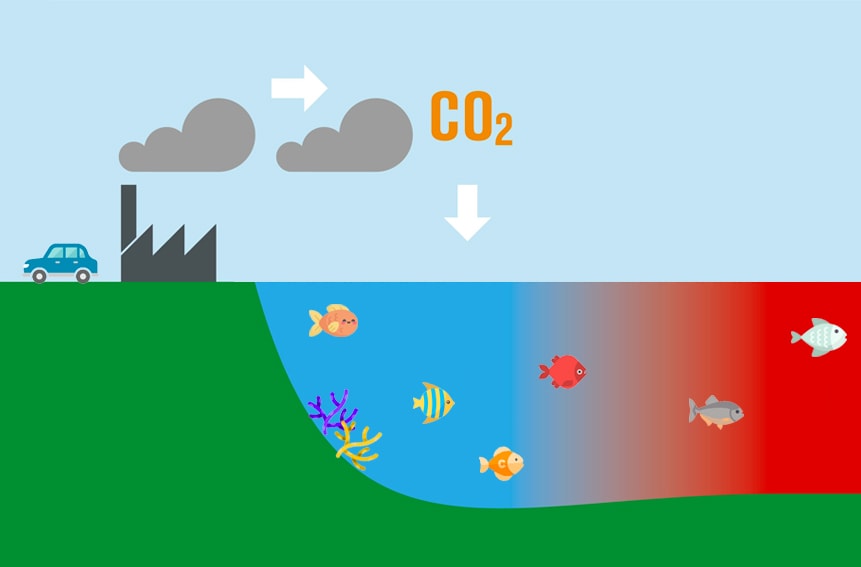
Since the industrial revolution, we have begun to burn a lot of fossil fuels. In doing so, we have released a lot of carbon dioxide (CO2) into the air.
As we mentioned earlier, we know that CO2 affects the atmosphere. But what most people don’t know, is that CO2 affects our oceans.
How?
Eventually…some of that CO2 falls back down into our oceans. How much?
Our oceans have absorbed about HALF of all human-produced CO2 emissions.
When CO2 dissolves in our oceans, it increases hydrogen ions. Those hydrogen ions, then bond with carbonate ions to form carbonic acid. This leads to a lower ocean pH level.
In other words…
When we pollute into the air, the ocean gets more acidic.
Take a look at the diagram below to see an illustration.
You see? The higher the CO2, the lower the ocean pH levels (lower pH, means more acidic).
Now let’s examine…
Sometimes called a “sea butterfly”, pteropods are tiny marine creatures. They’re actually about the size of a small pea.
While they may be small in stature, they are largely connected to the food web of the ocean.
Many fish, and even krill, have pteropods as part of their diet, or eat animals that eat pteropods.
Watch what happens to a pteropod’s shell after spending 45 days in sea water with a pH level of 7.8 (more on this later).
As you can see—almost overnight—the pteropod’s shell rapidly dissolved
As we mentioned earlier, due to CO2 pollution, carbonate ions are bonding with hydrogen ions.
Why is this important?
Because shellfish need carbonate ions to help form their protective shells made of calcium carbonate (CaCo3).
Shellfish include mollusks, crustaceans, sea-urchins, crabs, planktons and other marine species.
These species are being robbed of a fundamental building block necessary to their survival.
While fish aren’t don’t suffer the same dire consequences, they are still affected nonetheless.
They need to expel any excess acid. How? Through their gills, kidneys, and intestines. This process takes precious energy. Energy that could have been used to digest food or escape predators.
It also means fish sizes will get smaller.
Coral reefs are also composed of calcium carbonate. They use it to build their “hard skeleton”. This is especially true in the early stages of development.
With more carbonate ions bonding with hydrogen ions, that means less carbonate ions available for coral reefs.
The result?
A more brittle coral reef that has trouble reproducing.
Not only can entire coral reefs die, but the organisms that depend on it as well.
These species include red snapper and grouper.
We know CO2 pollution is getting worse. This also means, our ocean’s pH level will also continue to decrease (meaning more acidic).
Remember that pteropod shell we showed you dissolving?
That was in seawater with a pH level of 7.8.
As you can see above, scientists predict our ocean will reach a pH level of 7.8 in less than a century.
It would be nearly impossible to stop ocean acidification.
But what we can do is, slow it down. This will give our ocean a chance to acclimate to the new pH level.
So how we slow down ocean acidification?
By weening ourselves off fossil fuels.
This means using…
How big is your carbon footprint? Find out with this cool carbon footprint calculator.
This concludes our article ‘Learn About 5 Scary Consequences to Ocean Acidification’. If you enjoyed, please Like or Share on Facebook! Let us know in the comments below any clever ways you have been able to shrink your carbon footprint.
http://climateinterpreter.org/content/effects-ocean-acidification-marine-food-chain
http://climateinterpreter.org/content/effects-ocean-acidification-coral-reefs
http://ocean.si.edu/ocean-acidification
http://climateinterpreter.org/sites/default/files/resources/ocean_acid2_NMS.pdf
http://climateinterpreter.org/content/humans-can-take-action-slow-process-ocean-acidification
http://www.nature.org/greenliving/carboncalculator/index.htm?ref=www.nature.org